REPH Class Action Report
Levi & Korsinsky, LLP
June 7, 2018
On May 31, 2018, investors sued Recro Pharma, Inc., (“Recro” or the “Company”) in United States District Court, Eastern District of Pennsylvania. Plaintiffs in the federal securities class action allege that they acquired Recro stock at artificially inflated prices between July 31, 2017 and May 23, 2018 (the “Class Period”). They are now seeking compensation for financial losses incurred upon public revelation of the Company’s alleged misconduct during that time. Here’s everything you need to know about the Recro class action lawsuit (REPH class action lawsuit)
Summary of the Allegations
Company Background
The Company (NASDAQ: REPH) has been in business for 11 years and bills itself as a “a revenue-generating, specialty pharmaceutical company focused on products for hospitals and ambulatory care settings that is currently developing non-opioid products for the treatment of acute pain.”
As such, Recro receives royalties for the sales of five commercial products formulated at its own manufacturing facility.
The Company says its products are designed to eliminate the harmful side effects associated with opioid-based painkillers. However, Recro’s public statements about its lead product candidate, an injectable form of meloxicam (“IV meloxicam”), during the Class Period form the crux of the current lawsuit.
Summary of Facts
Recro and three of its top officers and/or directors now stand accused of deceiving investors by withholding and lying about the Company’s business, operational and compliance policies during the Class Period.
Specifically, they are accused of omitting truthful information about the FDA’s assessment of IV meloxicam from SEC filings and related material. By knowingly or recklessly doing so, they allegedly caused Recro stock to trade at artificially inflated prices during the time in question.
The truth emerged on May 24, 2018, when the Company announced that the FDA “had declined to approve Recro’s New Drug Application (“NDA”) for IV meloxicam. The Company also announced that it received a letter from the FDA in which the agency explained that, “the drug’s analgesic effects did not meet FDA expectations and raised questions related to chemistry, manufacturing and controls data.”
A closer look…
As alleged in the May 31 complaint, Recro repeatedly made misleading public statements throughout the Class Period.
For example, on a form filed with the SEC on August 11, 2017, the Company said in pertinent part: “Our lead product candidate, IV meloxicam, has successfully completed two pivotal Phase III clinical trials, a large Phase III safety trial and other safety studies for management moderate to sever pain.”
Then, in a press release issued September 29, 2017, an Individual Defendant in the current lawsuit said in pertinent part: “The acceptance for review of the IV meloxicam 30 mg NDA marks an important milestone in our effort to bring new therapeutics to patients and to the physicians who treat them.”
In another press release issued February 20, 2018, the Company stated in relevant part: “The data from this Phase 2 study support the growing body of clinical evidence demonstrating that IV meloxicam acts rapidly and offers durable pain relief, with a favorable safety profile.”
What the Company failed to disclose however, was that in reality, its lead product candidate “lacked supporting clinical data to show sufficient clinical benefits to receive FDA approval.”
Impact of the Alleged Fraud on Recro’s Stock Price and Market Capitalization
| Closing stock price prior to disclosures:
|
$12.42 |
| Closing stock price the trading day after disclosures:
|
$5.63 |
| One day stock price decrease (percentage) as a result of disclosures:
|
54.67% |
The following chart illustrates the stock price during the class period:
Actions You May Take
If you have purchased shares during the Class Period, you may join the class action as a lead plaintiff, remain a passive class member, or opt out of this litigation and pursue individual claims that may not be available to the class as a whole.
NOTE: The deadline to file for lead plaintiff in this class action is July 30, 2018. You must file an application to be appointed lead plaintiff prior to this deadline in order to be considered by the Court. Typically, the plaintiff or plaintiffs with the largest losses are appointed lead plaintiff.
In order to identify your potential exposure to the alleged fraud during the time in question, you may wish to perform an analysis of your transactions in Recro common stock using court approved loss calculation methods.
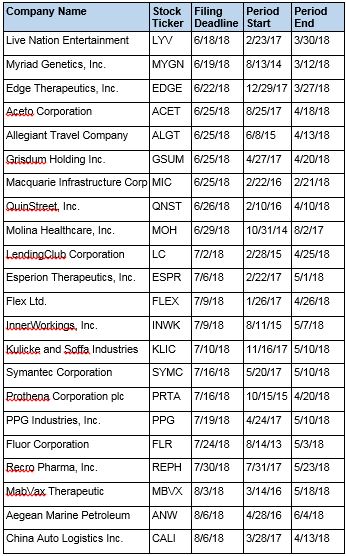
Recently Filed Cases
Listed below are recently filed securities class action cases being monitored by us, along with the class period and the deadline to file a motion to be appointed as the Lead Plaintiff in the action. Please contact us if you would like an LK report for any of these cases:
About Us
| This information is provided for general information purposes only, and should not be construed as legal advice, nor does it establish an attorney-client relationship with Levi & Korsinsky LLP. Any and all information herein is simply an opinion based on publicly available information and should not necessarily be construed as fact. For more information, please visit our website at www.zlk.com.
Attorney Advertising
|
Levi & Korsinsky is a leading securities litigation firm with a hard-earned reputation for protecting investors’ rights and recovering losses arising from fraud, mismanagement and corporate abuse. With thirty attorneys and offices in New York, Connecticut, California and Washington D.C., the firm is able to litigate cases in various jurisdictions in the U.S., England, and in other international jurisdictions.
Levi & Korsinsky provides portfolio monitoring services for high-net worth investors and institutional clients. Our firm also assists investors in evaluating whether to opt-out of large securities class actions to pursue individual claims.
For additional information about this case or our institutional services, please contact us.